COVID-19 IgG/IgM Rapid Test Cassette
Short Description:
The COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow immunoassay designed for the qualitative detection of IgG and IgM antibodies to the SARS-CoV-2 virus in whole blood, serum or plasma specimens from individuals suspected of COVID -19 infection by their healthcare provider .
The CO VID-19 IgG/IgM Rapid Test is an aid in the diagnosis of patients with suspected SARS -CoV-2 infection in conjunction with clinical presentation and the results of other laboratory tests. It is suggested to use as a supplementary test indicator for suspected cases with negative nucleic acid test of novel coronavirus or used in conjunction with nucleic acid test in suspected cases. Results from antibody testi ng should not be used as the sole basis to diagnose or exclude SARS -CoV-2 infection or to inform infection status.
Negative results do not rule out SARS -CoV-2 infection, particularly in those who have been in contact with known infected persons or in areas with high prevalence of active infection . Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
Positive results may be due to past or present infection with non -SARS- CoV-2 coronavirus strains .
The test is intended to be used at clinical laboratories or by healthcare workers at the point -of-care, not for home use. The test should not be used for screening of donated blood.
For professional and in vitro diagnostic use only.
For professional and in vitro diagnostic use only.
INTENDED USE
The COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow immunoassay designed for the qualitative detection of IgG and IgM antibodies to the SARS-CoV-2 virus in whole blood, serum or plasma specimens from individuals suspected of COVID -19 infection by their healthcare provider .
The CO VID-19 IgG/IgM Rapid Test is an aid in the diagnosis of patients with suspected SARS -CoV-2 infection in conjunction with clinical presentation and the results of other laboratory tests. It is suggested to use as a supplementary test indicator for suspected cases with negative nucleic acid test of novel coronavirus or used in conjunction with nucleic acid test in suspected cases. Results from antibody testi ng should not be used as the sole basis to diagnose or exclude SARS -CoV-2 infection or to inform infection status.
Negative results do not rule out SARS -CoV-2 infection, particularly in those who have been in contact with known infected persons or in areas with high prevalence of active infection . Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
Positive results may be due to past or present infection with non -SARS- CoV-2 coronavirus strains .
The test is intended to be used at clinical laboratories or by healthcare workers at the point -of-care, not for home use. The test should not be used for screening of donated blood.
SUMMARY
The novel coronaviruses belong to the p genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic injected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
When the SARS-CoV2 virus infects an organism, RNA, the genetic material of the virus, is the first marker that can be detected. The viral load profile of SARS-CoV-2 is similar to that of influenze, which peaks at around the time of symptom onset, and then begin to decline. With the development of the disease course after infection, the human immune system will produce antibodies, among which IgM is the early antibody produced by the body after infection, indicating the acute phase of infection. IgG antibodies to SARS-CoV2 become detectable later following infection. Positive results for both IgG and IgM could occur after infection and can be indicative of acute or recent infection. IgG indicates the convalescent phase of infection or a history of past infection.
However, both IgM and IgG have a window period from virus infection to antibody production, IgM almost appear after the onset of disease several days, so their detection often lags behind nucleic acid detection and is less sensitive than nucleic acid detection. In cases where nucleic acid amplification tests are negative and there is a strong epidemiological link to COVID-19 infection, paired serum samples (in the acute and convalescent phase) could support diagnosis.
PRINCIPLE
COVID-19 IgG/IgM Rapid Test Cassette (WB/S/P) is a qualitative membrane strip based immunoassay for the detection of antibodies (IgG and IgM) to Novel coronavirus in human Whole Blood/Serum/plasma. The test cassette consists of: 1) a burgundy colored coiyugate pad containing Novel coronavirus recombinant envelope antigens coi^ugated with Colloid gold (Novel coronavirus c两ugates), 2) a nitrocellulose membrane strip containing two test lines (IgG and IgM lines) and a control line (C line). The IgM line is pre-coated with the Mouse anti-Human IgM antibody, IgG line is coated Mouse anti-Human IgG antibody, when an adequate volume of lest specimen is dispensed into the sample well of the test cassette. The specimen migrates by capillary action across the cassette. IgM anti-Novel coronavirus, if present in the specimen, will bind to the Novel coronavirus coiyugates. The immunocomplex is then captured by the reagent pre-coated on the IgM band, forming a burgundy colored IgM line, indicating a Novel coronavirus IgM positive test result. IgG anti-Novel coronavirus it present in the specimen will bind to the Novel coronavirus conjugates. The immunocomplex is then captured by the reagent coated on lhe IgG line,forming a burgundy colored IgG line, indicating a Novel coronavirus IgG positive test result. Absence of any T lines (IgG and IgM) suggests a
negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
WARNINGS AND PRECAUTIONS
- For in vitro diagnostic use only.
- For healthcare professionals and professionals point of care sites.
•Do not use after the expiration date.
- please read all the information in this leaflet before performing the test. •The test cassette should remain in the sealed pouch until use.
•All specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
•The used test cassette should be discarded according to federal, state and local regulations.
COMPOSITION
The test contains a membrane strip coated with Mouse anti-Human IgM antibody and Mouse anti-Human IgG antibody on the
test line, and a dye pad which contains colloidal gold coupled with Novel corona virus recombinant antigen. The quantity of tests was printed on the labeling.
Materials Provided
- Test cassette • Package insert
- Buffer • Dropper
- Lancet
Materials Required But Not Provided
•Specimen collection container • Timer
STORAGE AND STABILITY
•Store as Packaged in the sealed pouch at the temperature(4-30″Cor 40-86°F).The kit is stable within the expiration date printed on the labeling.
•Once open the pouch, the lest should be used within one hour. Prolonged exposure to hot and humid environment will cause product deterioration.
•The LOT and the expiration date were printed on the labeling SPECIMEN
•The test can be used to test whole Blood/Serum/Plasma specimens.
•To collect whole blood, serum or plasma specimens following regular clinical laboratory procedures.
•Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear non-hemolyzed specimens.
•Store specimens at 2-8 °C (36-46T) if not tested immediately. Store specimens at 2-8 °C up to 7 days. The specimens should be frozen at -20 °C (-4°F) for longer storage. Do not freeze whole blood specimens、
•Avoid multiple freeze-thaw cycles, prior to testing, bring frozen specimens to room temperature slowly and mix gently.
Specimens containing visible particulate matter should be clarified by centrifugation before testing.
•Do not use samples demonstrating gross lipemia gross hemolysis or turbidity in order to avoid interference on result interpretation
TEST PROCEDURE
Allow the test device and specimens to equilibrate to temperature(15-30 C or 59-86 T ) prior to testing.
- Remove the test Cassette from the sealed pouch.
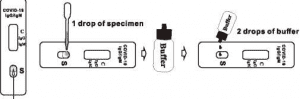
- Hold the dropper vertically and transfer 1 drop (approximately 10 ul) of specimen into the upper area of the specimen well(S) making sure that there are no air bubbles. For better precision, transfer specimen by a pipette capable of delivering 10 ul of volume. See the illustration below.
- Then, add 2 drops(approximately 70 ul) of buffer immediately into the specimen well(S).
- Start the timer.
- for colored lines to appear. Interpret the test results at 15 minutes. Do not read results after 20 minutes.
Area for Specimen
(The picture is for reference only, please refer to the material object.)
INTERPRETATION OF RESULTS
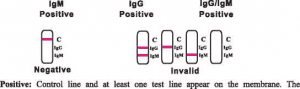
antibodies. The appearance of IgM test line indicates the presence of Novel coronavirus specific IgM antibodies. And if both IgG and IgM line appear, it indicates that the presence of both Novel coronavirus specific IgG and IgM antibodies.
Negative: One colored line appears in the control region(C), No apparent colored line appears in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons fbr control linefailure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
QUALITY CONTROL
A procedural control is included in the test. A colored line appearing in the control region (C) is considered an internal procedural control. It confirms sufficient specimen volume, adequate membrane wicking and correct procedural technique. Control standards are not supplied with this kit. However, it is recommended that positive and negative controls be tested as good laboratory practice to confirm the test procedure and to verify proper test performance.
LIMITATIONS
• COVID-19 IgG/IgM Rapid Test Cassette (WB/S/P) is limited to provide a qualitative
detection. The intensity of the test line does not necessarily correlate to the concentration of the antibody in the blood. The results obtained from this test are intended to be an aid in diagnosis only. Each physician must interpret the results in conjunction with the patient’s history, physical findings, and other diagnostic procedures.
•A negative test result indicates that antibodies to Novel coronavirus are either not present or at levels undetectable by the test.
PERFORMANCE CHARACTERISTICS
Accuracy
Summary data of CO VID-19 IgG/IgM Rapid Test as below
Regarding the IgG test, we have counted the positive rate of the 82 patients during the convalescence period.
COVID-19 IgG:
The results yielding a sensitivity of 97.56%
Regarding the IgM test, the result comparison to RT-PCR.
COVID-19 IgM:
A statistical comparison was made between the results yielding a sensitivity of 88.61 %, a specificity of 97.67% and an accuracy of 93.33%
Cross-Reactivity and Interference
1 .Other common causative agents of infectious diseases were evaluated for cross reactivity with the test. Some positive specimens of other common infectious diseases were spiked into the Novel coronavirus positive and negative specimens an d tested separately. No cross reactivity was observed with specimens from patients infected with HIV,HA^ HBsAg, HCV TP, HTIA^ CMV FLUA, FLUB, RSy MP, CP, HPIVs.
2.Potentially cross-reactive endogenous substances including common serum components, such as lipids, hemoglobin, bilirubin, were spiked at high concentrations into the Novel coronavirus positive and negative specimens and tested, separately.
No cross reactivity or interference was observed to the device.
3.Some other common biological analytes were spiked into the Novel coronavirus positive and negative specimens and tested separately. No significant interference was observed at the levels listed in the table below.
Reproducibility
Reproducibility studies were performed for Novel coronavirus IgG/IgM Rapid Test at three physician office laboratories (POL). Sixty (60) clinical serum specimens, 20 negative, 20 borderline positive and 20 positive, were used in this study. Each specimen was run in triplicate for three days at each POL. The intra-assay agreem ents were 100%. The inter-site agreement was 100 %.