SARS-CoV-2 Antigen Rapid Test Cassette
Takaitaccen Bayani:
The SARS-CoV-2 Antigen Rapid Cassette ne mai saurin chromatographic immunoassay don gano ingantattun antigen SARS-CoV-2 a cikin swabs na Oropharyngeal na ɗan adam. Gano yana dogara ne akan ƙwayoyin rigakafi na monoclonal takamaiman na Protein Nucleocapsid (N) na SARS- CoV-2.An yi niyya don taimakawa cikin saurin ganewar cutar COVID-19.
AMFANI DA NUFIN
TheSARS-CoV-2 Antigen Rapid Test CassetteImmunoassay ne mai saurin chromatographic don gano ƙimar ingancin SARS-CoV-2 antigen a cikin swabs na Oropharyngeal na mutum. An gano shi ne akan ƙwayoyin rigakafi na monoclonal musamman na Nucleocapsid (N) Protein na SARS-CoV-2.An yi nufin taimakawa a cikin da sauri bambancin ganewar asali naCUTAR COVID 19kamuwa da cuta.
Bayanin Kunshin
Gwaje-gwaje 25 / fakiti, gwaji 50 / fakiti, gwaji 100 / fakiti
GABATARWA
Novel coronaviruses na β jinsi ne.CUTAR COVID 19cuta ce mai saurin kamuwa da cutar numfashi.Mutane gaba daya suna da saukin kamuwa.A halin yanzu, marasa lafiya da suka kamu da novel coronavirus sune babban tushen kamuwa da cuta, masu kamuwa da asymptomatic suma suna iya zama tushen kamuwa da cuta.Binciken da ake yi na cututtukan cututtuka na yanzu, lokacin shiryawa shine 1. zuwa kwanaki 14, yawanci kwanaki 3 zuwa 7.Babban bayyanar cututtuka sun haɗa da zazzabi, gajiya da bushewar tari.Ana samun cunkoso na hanci, na hanci, ciwon makogwaro, myalgia da gudawa a wasu lokuta.
REAGENTS
Kaset ɗin gwajin ya ƙunshi anti-SARS-CoV-2 Nucleocapsid protein barbashi da anti-SARS-CoV-2 Nucleocapsid protein rufi a kan membrane.
MATAKAN KARIYA
Da fatan za a karanta duk bayanan da ke cikin wannan kunshin kafin yin gwajin.
1.Don ƙwararrun in vitro diagnostic amfani kawai.Kada ku yi amfani da bayan ranar karewa.
2.Ya kamata gwajin ya kasance a cikin jakar da aka rufe har sai an shirya don amfani.
3.Duk samfurori yakamata a yi la'akari da su masu haɗari kuma a sarrafa su kamar yadda wakilin kamuwa da cuta.
4.Ya kamata a jefar da gwajin da aka yi amfani da shi bisa ga ka'idodin gida.
5.A guji amfani da samfuran jini.
6.Wear safofin hannu wen mika da samfurori, kauce wa taba da reagent membrane da samfurin da kyau.
AJIYA DA KWANTA
Lokacin tabbatarwa shine watanni 18 idan an adana wannan samfurin a cikin yanayin
2-30℃.The gwajin ne barga ta hanyar ranar karewa buga a kan shãfe haske jaka.The gwajin dole ne ya kasance a cikin shãfe haske jaka har sai da amfani..KADA KA DAKE.Kar a yi amfani da bayan ranar karewa.
TATTAUNAWA MISALIN DA SHIRI
1.Tarin ɓoye maƙogwaro: Saka swab maras kyau a cikin makogwaro gaba ɗaya daga baki, yana maido da bangon makogwaro da wurin jajayen tonsils na palate, goge tonsils na pharyngeal biyu da bangon pharyngeal na baya tare da matsakaici.
da karfi, kauce wa taba harshe da fitar da swab.
2.Process samfurin nan da nan tare da samfurin cirewar samfurin da aka bayar a cikin kit bayan an tattara samfurin.Idan ba za a iya sarrafa shi nan da nan ba, samfurin ya kamata a adana shi a bushe, haifuwa da bututun filastik da aka rufe sosai.Ana iya adana shi a 2-8 ℃ na tsawon sa'o'i 8, kuma ana iya adana shi na dogon lokaci a -70 ℃.
3. Samfuran da suka gurɓata sosai ta ragowar abinci na baka ba za a iya amfani da su don gwada wannan samfurin ba.Samfuran da aka tattara daga swabs waɗanda ke da ɗan ƙoƙon ƙoƙo ko ƙaranci ba a ba da shawarar gwada wannan samfurin ba.Idan swabs sun gurbata da adadi mai yawa na jini, ba a ba da shawarar su don gwaji ba.Ba a ba da shawarar yin amfani da samfuran da aka sarrafa tare da samfurin hakar samfurin da ba a bayar da su a cikin wannan kit ɗin don gwada wannan samfurin ba.
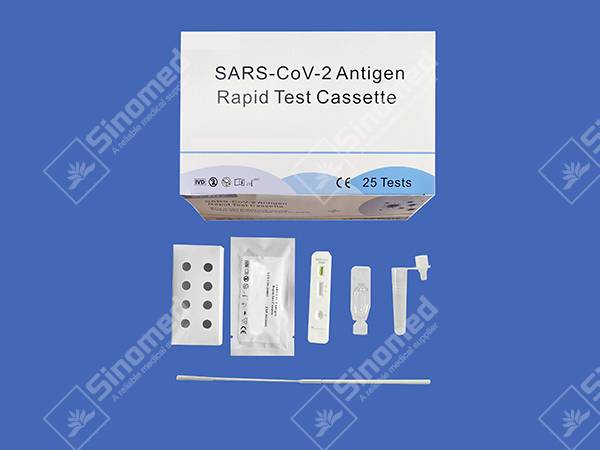
KIT ABUBUWAN
Kayayyakin samarwa
| Gwada kaset | Extraction Reagent | Bututun cirewa | |
| Bakararre Swabs | Saka Kunshin | Tashar Aiki |
Abubuwan da ake buƙata amma ba a bayarwa ba
| Mai ƙidayar lokaci | Don amfani da lokaci. |
| Kunshin |
Takaddun bayanai25
gwaje-gwaje/pack50
gwaje-gwaje/pack100
gwaje-gwaje / fakitiSample hakar Reagent25 gwaje-gwaje/pack50 gwaje-gwaje/pack100 gwaje-gwaje/packSample hakar
tube≥25 gwaje-gwaje/pack≥50 gwaje-gwaje/pack≥100 gwaje-gwaje/packInstructionKoma zuwa ga
kunshin koma zuwa ga
kunshin koma zuwa ga
kunshin
HANYOYIN AMFANI
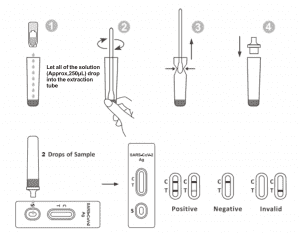
Bada gwajin, samfuri, buffer cirewa don daidaitawa zuwa zafin jiki (15-30 ℃) kafin gwaji.
1.Cire kaset ɗin gwajin daga jakar da aka rufe kuma a yi amfani da shi cikin mintuna 15.Za a sami sakamako mafi kyau idan an yi gwajin nan da nan bayan buɗe jakar jakar.
2.Place da Extraction Tube a cikin aikin tashar. Rike da hakar reagent kwalban juye a tsaye.Matsi da kwalban da kuma bari duk na bayani (Kimanin,250μL) sauke a cikin hakar tube da yardar kaina ba tare da shãfe gefen tube zuwa hakar. Tube.
3. Sanya samfurin swab a cikin Tube Extraction. Juya swab na kimanin daƙiƙa 10 yayin da kake danna kai a cikin bututu don saki antigen a cikin swab.
4. Cire swab yayin da kake matse kan swab a cikin bututun hakar yayin da kake cire shi don fitar da ruwa mai yawa gwargwadon yuwuwar samar da swab. Yi watsi da swab daidai da ka'idar zubar da shara ta biohazard.
5.Fit the dropper tip a saman bututun hakar. Sanya kaset ɗin gwajin a kan tsaftataccen wuri mai tsabta.
6.Add 2 saukad da mafita (kimanin, 65μL) zuwa samfurin da kyau sannan kuma fara mai ƙidayar lokaci. Karanta sakamakon da aka nuna a cikin minti 20-30, kuma sakamakon da aka karanta bayan minti 30 ba shi da inganci.
FASSARAR SAKAMAKO
| MARA SAKAMAKO: |
Layi mai launi ɗaya yana bayyana a yankin layin sarrafawa (C).Babu wani layi da ya bayyana a yankin gwajin (T).Sakamako mara kyau yana nuna cewa SARS-CoV-2 antigen baya cikin samfurin, ko kuma yana ƙasa da matakin da ake iya ganowa na gwajin.
KYAUTASAKAMAKO:
Layuka biyu sun bayyana.Layi mai launi ɗaya yakamata ya kasance a cikin yankin sarrafawa (C) kuma wani layin launi na fili yakamata ya kasance a cikin yankin gwaji (T) sakamako mai kyau yana nuna cewa an gano SARS-CoV-2 a cikin samfurin.
Sakamako mara inganci:
Layin sarrafawa ya kasa bayyana. Rashin isassun ƙarar samfurin ko dabarun tsari da ba daidai ba shine mafi kusantar dalilai na gazawar layin sarrafawa.Bita tsarin kuma maimaita gwajin tare da sabon gwaji.Idan matsalar ta ci gaba, daina amfani da kayan gwajin nan da nan kuma tuntuɓi mai rarrabawa na gida.
NOTE:
Ƙarfin launi a yankin layin gwaji (T) zai bambanta dangane da tattarawar SARS-CoV-2 Antigen da ke cikin samfurin.Saboda haka, duk wani inuwa na launi a cikin yankin layin gwaji (T) ya kamata a yi la'akari da shi mai kyau.
KYAUTATA KYAUTA
- An haɗa tsarin sarrafawa a cikin gwajin.Layi mai launi da ke bayyana a yankin sarrafawa (C) ana ɗaukarsa a matsayin kulawar tsari na ciki. Yana tabbatar da isassun wicking membrane.
- Ba a samar da matakan sarrafawa tare da wannan kit;duk da haka, ana ba da shawarar cewa a gwada sarrafawa mai kyau da mara kyau a matsayin kyakkyawan aikin dakin gwaje-gwaje don tabbatar da tsarin gwajin da kuma tabbatar da aikin gwajin da ya dace.
IYAKANA GWAJI
- TheSARS-CoV-2 Antigen Rapid Test Cassetteƙwararre ce kawai don amfani da bincike na in vitro kawai.Ya kamata a yi amfani da gwajin don gano SARS-CoV-2 Antigen a cikin Oropharyngeal Swab. Ba za a iya ƙayyade ƙimar ƙima ko ƙimar haɓakar ƙwayar SARS-CoV-2 ta wannan ƙimar. gwadawa.
- Daidaiton gwajin ya dogara da ingancin samfurin swab. Ƙarya mara kyau na iya haifar da samar da samfurin tarawa mara kyau.
- Cassette ɗin gwajin sauri na SARS-CoV-2 Antigen Rapid zai nuna kawai kasancewar SARS-CoV-2 a cikin samfurin daga nau'ikan coronavirus na SARS-CoV-2 masu ƙarfi da marasa ƙarfi.
- Kamar yadda yake tare da duk gwaje-gwajen bincike, duk sakamakon dole ne a fassara shi tare da wasu bayanan asibiti da ke akwai ga likita.
- Ya kamata PCR ya tabbatar da mummunan sakamako da aka samu daga wannan kit ɗin. Za a iya samun mummunan sakamako idan tarin SARS-CoV-2 da ke cikin swab bai isa ba ko kuma yana ƙasa da matakin gwajin da ake iya ganowa.
- Yawan jini ko gamsai akan samfurin swab na iya tsoma baki tare da aiki kuma yana iya haifar da sakamako mai kyau na ƙarya.
- Kyakkyawan sakamako ga SARS-CoV-2 baya hana kamuwa da kamuwa da cuta tare da anther pathogen.Don haka yakamata a yi la'akari da yuwuwar kamuwa da cutar kwayan cuta.
- Sakamako mara kyau baya kawar da kamuwa da cutar SARS-CoV-2, musamman a cikin waɗanda suka yi mu'amala da kwayar cutar.Ya kamata a yi la'akari da gwajin gwaji tare da binciken kwayoyin don kawar da kamuwa da cuta a cikin waɗannan mutane.
- Kyakkyawan sakamako na iya kasancewa saboda kamuwa da cuta tare da nau'ikan coronavirus marasa SARS-CoV-2, kamar coronavirus HKU1, NL63, OC43, ko 229E.
- Sakamakon gwajin antigen bai kamata a yi amfani da shi azaman tushen tushe kawai don ganowa ko ware kamuwa da cutar SARS-CoV-2 ko sanar da matsayin kamuwa da cuta ba.
- Extraction reagent yana da ikon kashe kwayar cutar , amma ba zai iya kunna 100% na kwayar cutar ba. Hanyar inactivating kwayar cutar za a iya koma zuwa: wace hanya ce ta WHO/CDC, ko za a iya sarrafa ta bisa ga dokokin gida.
HALAYEN YI
HankalikumaMusamman
An kimanta kaset ɗin gwajin sauri na Antigen na SARS-CoV-2 tare da samfuran samfuran da aka samo daga marasa lafiya. Ana amfani da PCR azaman hanyar tunani don SARS-CoV-2 Antigen Rapid Cassette. Ana ɗaukar samfuran inganci idan PCR ya nuna sakamako mai kyau.
| Hanya | RT-PCR | Jimlar Sakamako | ||
| SARS-CoV-2 Antigen Rapid Test Cassette | Sakamako | M | Korau | |
| M | 38 | 3 | 41 | |
| Korau | 2 | 360 | 362 | |
| Jimlar Sakamako | 40 | 363 | 403 | |
Hankalin Dangi:95.0%(95%CI*:83.1%-99.4%)
Ƙimar Dangi:99.2%(95%CI*:97.6%-99.8%)
*Tazarar Amincewa
Iyakar Ganewa
Lokacin da abun cikin ƙwayoyin cuta ya fi 400TCID50/ml, ingantaccen ƙimar ganowa ya fi 95%.Lokacin da abun cikin ƙwayoyin cuta bai wuce 200TCID ba50/ml, ingantaccen ƙimar ganowa bai wuce 95% ba, don haka mafi ƙarancin gano wannan samfurin shine 400TCID50/ml.
Daidaitawa
An gwada batches uku a jere na reagents don daidaito.An yi amfani da batches daban-daban na reagents don gwada samfurin mara kyau sau 10 a jere, kuma sakamakon duka mara kyau ne.An yi amfani da batches daban-daban na reagents don gwada samfurin tabbatacce sau 10 a jere, kuma sakamakon duka tabbatacce ne.
Tasirin HOOK
Lokacin da abun ciki na ƙwayoyin cuta a cikin samfurin da za a gwada ya kai 4.0*105TCID50/ml, sakamakon gwajin har yanzu bai nuna tasirin HOOK ba.
Cross-Reactivity
An ƙididdige sake kunnawar Kit ɗin.Sakamako ya nuna babu giciye mai aiki tare da samfurori masu zuwa.
| Suna | Hankali |
| HCOV-HKU1 | 105TCID50/ml |
| Staphylococcus aureus | 106TCID50/ml |
| Rukunin A streptococci | 106TCID50/ml |
| Kwayar cutar kyanda | 105TCID50/ml |
| Cutar mumps | 105TCID50/ml |
| Adenovirus nau'in 3 | 105TCID50/ml |
| Mycoplasmal ciwon huhu | 106TCID50/ml |
| Paraimfluenzavirus, nau'in 2 | 105TCID50/ml |
| Mutum metapneumovirus | 105TCID50/ml |
| Mutum coronavirus OC43 | 105TCID50/ml |
| Mutum coronavirus 229E | 105TCID50/ml |
| Bordetella parapertusosis | 106TCID50/ml |
| mura B Victoria STRAIN | 105TCID50/ml |
| mura B YSTRAIN | 105TCID50/ml |
| mura A H1N1 2009 | 105TCID50/ml |
| Mura A H3N2 | 105TCID50/ml |
| H7N9 | 105TCID50/ml |
| H5N1 | 105TCID50/ml |
| Epstein-Barr cutar | 105TCID50/ml |
| Enterovirus CA16 | 105TCID50/ml |
| Rhinovirus | 105TCID50/ml |
| Ƙwayar cutar da ke kama huhu | 105TCID50/ml |
| Streptococcus pneumoni-ae | 106TCID50/ml |
| Candida albicans | 106TCID50/ml |
| Chlamydia pneumoniae | 106TCID50/ml |
| Bordetella pertussis | 106TCID50/ml |
| Pneumocystis jijiya | 106TCID50/ml |
| Mycobacterium tuberculosis | 106TCID50/ml |
| Legionella pneumophila | 106TCID50/ml |
IAbubuwan da ke kawo cikas
Sakamakon gwajin ba za a tsoma baki tare da abun da ke cikin abubuwan da ke gaba ba:
| Tsangwama abu | Conc. | Abu mai shiga tsakani | Conc. |
| Dukan Jini | 4% | Benzoin Gel | 1.5mg/ml |
| Ibuprofen | 1 mg/ml | Cromolyn glycate | 15% |
| tetracycline | 3 ug/ml | chloramphenicol | 3 ug/ml |
| Mucin | 0.5% | Mupirocin | 10mg/ml |
| Erythromycin | 3 ug/ml | Oseltamivir | 5mg/ml |
| Tobramycin | 5% | Naphazoline Hydrochlo-hau Hanci Drops | 15% |
| menthol | 15% | Fluticasone propionate | 15% |
| Afrin | 15% | Deoxyepinephrine hydro-chloride | 15% |
IBBLIOGRAPHY
1.Weiss SR,Leibowitz JZ.Coronavirus pathogenesis.Adv Virus Res 2011;81:85-164
2.Cui J,Li F,Shi ZL.Asalin da kuma juyin halitta na pathogenic coronaviruses.Nat Rev Microbiol 2020;
3.Su S, Wong G, Shi W, et al. Epidemiology, sake hadewar kwayoyin halitta, da kuma pathogenesis na coronaviruses.TrendsMicrobiol 2016;24:490-502.